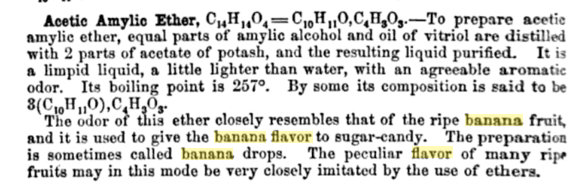The still-catchy tune "Yes! We have no bananas" dates from an earlier banana extinction scare in the 1920s. (Image from NYPL.)
Have you heard? Bananas are going extinct!
Don't worry; this has happened before.
For the first half of the twentieth century, Americans were eating a different type of banana: the Gros Michel. (Fat Mike, to its friends.) Native to the Americas, Gros Michel was grown in massive plantations in Honduras, Costa Rica, and elsewhere in Central America, most of which were owned by a few huge companies. But by the 1950s, fungal diseases had ravaged production, destroying more than a hundred thousand acres of Central American banana plantations.
The Gros Michel was replaced by a banana of Asian origin, the Cavendish, which was resistant to the fungal blights that had wreaked havoc on its predecessor. Predictably, the story has now repeated itself. Intensive monoculture and the interconnectedness of global trade virtually assures the spread of pathogens, wrecking crops, devastating local banana economies. In the end, fungus always wins.
You may have also heard the persistent rumor that, banana to banana, the Gros Michel bested the Cavendish in every way. "Fifty years ago, we were eating better bananas," broods CNN. According to the somber assessments of these banana moralists, the Cavendish is blander, more boring, needs "artificial" ripening, is altogether more buttoned-up and tucked-in than the wilder, fruitier Fat Mike.
There's another rumor: If you want a hint of what the Gros Michel tasted like, try a banana Laffy Taffy, or those little yellow banana candies, or any cheap banana-flavored thing. Fake banana flavor, the legend goes, is based on the Gros Michel. There's some evidence that isoamyl acetate — banana ester, the characterizing component of "fake" banana flavors — was a more prominent note in the Gros Michel than it is in the Cavendish.
Good old New England Confectionery Company chewy banana splits
"It's not that the fake banana flavor doesn’t taste like bananas, it’s that bananas don’t taste as flavorful as they used to," concludes a recent article about fake-banana-real-banana on foodandwine.com.
So this is what we are left with: an apparitional Gros Michel. "Fake banana" flavor, a shabby memento of a better, more delicious banana that was wiped from the planet (or, at least, the export economy) by the hubris of industrial agriculture. Modernity always promises us better living, but meanwhile perpetrates these secret swaps, leaving us with mass-produced versions of nature: duller, dimmer, less.
Or at least this is a story that we like to tell ourselves — that the price we pay for living the way we do, allegedly unconstrained by nature, is that we are consequently denied our full measure of experience. As we pass into the future, we get worse and worse bananas.
But was "fake banana" flavor really "based" on the Gros Michel? Was the Gros Michel better? Is the fake inevitably an attenuation of the real? What is "real" banana flavor, anyways?
And could it even be possible that fake banana flavor came before real bananas?
Let's not get ahead of ourselves. Let's begin with the bananas.
According to John Soluri, whose excellent Banana Cultures: Agriculture, Consumption, and Environmental Change in Honduras and the United States I'm drawing on here for most of these banana facts, prior to the 1850s, bananas were rare indeed in these United States.
And most Americans wouldn't get a taste of bananas until the 1876 Centennial Exhibition in Philadelphia, where the fruit, wrapped in foil and sold for a dime, drew gigantic crowds. At first, multiple varieties of bananas were available in US markets, red and yellow, but by the 1890s, one banana reigns supreme: the Gros Michel.
Stereogram of banana trees on display at the 1876 Philadelphia Centennial Exhibition.
There are many reasons that Gros Michel became the top banana. Superior taste was by no means the main factor here. (After all, prior to a consumer market in bananas, how can we know what people believe the best-tasting banana to be?) In fact, the features that put Gros Michel squarely on top had to do with logistics — the logistics of getting bananas from Central America to U.S. ports and then to markets in the late nineteenth and early twentieth centuries, i.e., by train and by boat.
Gros Michel were thick-skinned, resistant to bruising. A bunch of Gros Michel bananas tended to include more "hands" (that's the term of individual bananas) than other varietals, and those bunches basically packed themselves: the hands grew tight and symmetrical, perfect for tossing straight into a ship's cargo hold. The bananas were thick-skinned, resistant to bruising, and had a long ripening period, and grocers appreciated their attractive, unblemished bright yellow appearance. Basically, Gros Michel bananas were born to be shipped.
By the 1890s, most bunches of banana entering the U.S. were yellow Gros Michel bananas, "the variety around which late-nineteenth-century consumer markets formed their notions about just what constituted a 'banana,'" according to Soluri.
This 1917 photograph by Lewis Hine shows a boy peddling bananas in Boston. Image courtesy Library of Congress.
And so, in 1912, when Clemens Kleber, head chemist for the flavor and fragrance firm Fritzsche Brothers, set out to determine which chemicals in bananas were responsible for their flavor, the bananas that he used in his New Jersey research laboratory were, almost certainly, Gros Michel.
After ripening, mashing, distilling, and variously analyzing his banana mush, Kleber managed to isolate a quantity of an oily, odorous, neutral liquid, which he identified as amyl acetate.
[Note/plea to chemists: I know that isoamyl acetate and amyl acetate are different molecules. But I've found references that indicate that this difference was less significant to nineteenth-century and early-twentieth century chemists. For instance, this 1894 chemical dictionary presents the two as synonymous. Not being a chemist, I don't quite know what to make of this. What difference does the difference between these two molecules make? In what processes, reactions, and applications are they not interchangeable?]
Milt Gross, pioneering cartoonist, illustrating the real meaning of "banana oil!" (ie, bullshit.)
Kleber's motive for studying the chemical constituents of banana was, in part, to challenge the principles of the 1906 Pure Food and Drug law, which required flavor extracts containing synthetic chemicals to be labeled as "imitation." But if the chemicals used in preparing a synthetic flavor were the same as those present in the actual fruit, how could regulatory officials tell the difference? And why should labels impose a difference that did not exist (according to Kleber) on the molecular level? "As the evidence that substances identical with the so called artificial fruit ethers are also present in natural fruit flavors is of considerable importance in reference to the various pure food laws, I intend to make further researches about the composition of other natural fruit flavors," he vowed, in the December 1912 article where he described his banana research, continuing "It is, however, by no means my intention to monopolize this field of research" — and he certainly appears not to, as he never published anything of the sort again.
As was the case with methyl anthranilate and grape flavor, the reason that amyl acetate was used as banana flavor is not because chemists already knew that it as a banana-native substance. In fact, in order to really understand where artificial banana flavor comes from, you have to start with artificial pear. Because amyl acetate — produced from fusel oil, a waste product of alcohol distilling, and one of the very first synthetic chemicals used as an artificial flavor -- initially came to prominence as a pear flavoring.
Pear drops — barley sugar flavored with amyl acetate diluted in alcohol — were one of the new confections available at the 1851 Crystal Palace exhibition in London. The drops and the chemical used to flavor them drew the attention of August Hofmann, the distinguished chemist who was one of the judges of the exhibition. In a letter to Justus Liebig, his teacher, he noted the "remarkably fruity odor" of amyl acetate, and the "agreeable odour of the Jargonelle pear" that emerged when it was diluted in alcohol. Upon inquiry, he learned that "tolerably large quantities" of amyl acetate were being manufactured. "It is principally used for flavoring pear drops, which are much admired in England."
Jargonelle pears are an early-ripening pear common in Great Britain, but (it seems) relatively rare in the United States. And pear drop candies are also more common across the pond. According to Wikipedia, "A 2009 survey of 4,000 adults found that pear drops were the fourteenth most popular sweet in the United Kingdom."
Chemical catalogs from the 1850s through 1880s often refer to amyl acetate as "pear oil" or "jargonelle pear essence." But as the twentieth century nears, in the United States, the chemical is increasingly referred to as "banana oil," not only in flavor and fragrance raw material catalogs, but also in materials that refer to amyl acetate's other uses (especially as a paint thinner or varnish remover.)
So this is the story I originally wanted to tell here. I wanted to show that amyl acetate first signified the flavor of pears — was tagged, specifically, to jargonelle pears — then, in the United States, came to signify the flavor of bananas. I wanted to use this to show that our association between a sensory experience produced by a chemical and a particular real-world referent is historical, contingent, socially constructed. What amyl acetate reminds you of depends on your experiences and your frame of reference.
I wanted to tell that story, but then I dug a little deeper, and I discovered that the historical record doesn't support that hypothesis as tidily as I'd hoped. The past is a messy place! And a more interesting place than we perhaps imagine.
Working on a draft of my first chapter, I was reviewing a handful of notices from the early 1850s advertising "fruit essences," ie artificial fruit flavors, in Philadelphia, New York, and Boston newspapers.
And I was surprised — shocked, even — to find "banana" listed among the flavors offered, as early as 1855. Looking closer, it seems that banana flavor was present at the Crystal Palace as well. Scientific American, in its 1853 review of the exhibition's highlights, featured an account of the new artificial fruit essences, and claimed that the most common flavors at the exhibition were pineapple and banana. (Is it any accident that, in contrast to the other available flavors — jargonelle pear, greengage plum, apple — these are both "exotic" fruits, fruits we can assume many of the visitors to the exhibition had never had the opportunity to taste in the flesh?)
What comprised banana essence? The earliest formula I've found dates from 1859, from an important American textbook for pharmacists, which describes the composition of some of the "most prominent" commercially available artificial flavors. "Banana essence" is there described as a mixture of amyl acetate and "some" butyric ether, diluted in alcohol. (The book gives the formula for jargonelle pear as amyl acetate, diluted in alcohol. I should also note here that amyl acetate was a component of many synthetic fruit flavors in this period, not just pear and banana.)
Edward Kent, a manufacturer, importer, and dealer of chemicals and other chemical supplies, lists amyl acetate alternately as "Banana Essence" in his 1854 catalogue. But another New York chemical supply dealer, J.F. Luhme, lists amyl acetate as "pear oil" in a catalogue from the same period. What accounts for the difference? I'm not certain. However, while Luhme was only an importer, Kent was also a manufacturer -- ie, his company was making some of these substances in-house. Could a (relatively?) greater banana-consciousness in the U.S. at the time summon that fruit first to mind, prior to the pear?
Image from a chemistry textbook from 1860, published in Philadelphia, that associates amyl acetate with banana, not jargonelle pear. Digitized by Googlebooks.
In 1879, an article in a Canadian pharmaceutical journal reprinting Kletzinsky's flavor formulas makes an addition: "essence of banana," a flavor absent from Kletzinsky's table, but "much employed in the United States." The author indicates that it usually comprises equal parts of amyl acetate and ethyl butyrate, combined with five parts of alcohol.
So what arrived first to the American sensorium, banana flavor or bananas? Most people writing about the history of bananas in the US seem to agree that the fruit is rather rare and precious prior to the late 1870s. It seems that amyl-acetate-based banana flavor had a peak in popularity that anticipated or slightly preceded the widespread availability of Gros Michel bananas. Perhaps the presence of banana flavors in confections, beverages, and candies conditioned Americans to expect certain sensory qualities when it came to the taste of bananas, familiarized them with certain aspects of banana flavorness that they then were able to find and confirm in the Gros Michel.
Because of course, multiple chemicals contribute to the flavor of bananas, whether Gros Michel, Cavendish, or any of the hundreds of other banana varietals — green, blue, red, pink, and yellow — that grow in bunches on this wonderful planet we seem on the verge of wrecking forever. And we learn to attend to certain sensations in the multiplicity of sensation, and to mark them as the significant ones — to recognize and know the flavor of banana in amyl acetate. In a certain manner of speaking we are always denied our full measure of experience, because perception is always selective; the sensations we attend to, and the meanings we attach to them, are shaped by our histories and the contexts in which we live.
When making a banana flavor today, flavor chemists have access not only to a more exhaustive literature of the multiple chemicals that contribute to the flavor of bananas, but also to a far wider range of synthetic chemicals. But a "better" banana flavor is not always one that's more "real." Instead, flavorists build situational bananas, tailored to the food the flavor will be used in, the requirements of the market, and expectations and desires of consumers — also perhaps to something else, a different note, a new sensory idea. (If I've accomplished anything with this blog, I hope it's to shake up the belief that flavors should be bounded by some materialist, literal version of reality; or that questions of quality and pleasure can be settled by drawing a line between the "artificial" and the "genuine.")
But seriously — how "real" is a banana, anyways? (I should probably take this opportunity to assure everyone that bananas aren't going extinct, though the identity of the "banana of commerce" may be revised.)
Chiquita banana ad from 1970 that I found on the internet (and now can't find the source of), demonstrating the fruit's considerable potential as a cross-branding platform.
After all, the commercial banana shares many of the features that characterize the kind of food that we think of as industrial, mass-produced. Cheap and sweet, the banana was the first fresh fruit available for mass consumption in the U.S. that was available all year round. It's always banana season. The monocultural cultivation of a single banana varietal offers a kind of global uniformity reminiscent of Coca-Cola or Oreos. Bananas even come in their own packages, with surfaces susceptible to brand names, logos, and other inducements.
I want to end here by invoking one final role played by the banana in the early twentieth-century. T.H. Morgan's fruit fly lab at Columbia University is a crucial site in the history of science, the place where, at the beginning of the twentieth century, the foundations of modern genetics were laid.
In Morgan's lab, the fruit fly, cheap, brief, and prolific, was made into a "living instrument" to sustain the argument, provide the proof, of the connection between genes and traits, the chromosomal theory of heredity.
And what sustained Morgan's flies? Bananas. Cheap, abundant, always available, bananas were the model food for the first model organism, the insect whose cells would be used to map out the patterns of genes, at the moment when genes first seemed to be the stuff that makes our selves.
Bananas hang in bunches in Thomas Hunt Morgan's fly room, Columbia University, c. 1920.
Time flies like an arrow, fruit flies like a banana — and apparently, so do we.